The simplification and modification of the structure of natural flavolignans and flavonoids, as well as the introduction of different functional groups into the molecules of their synthetic analogs, as well as the change in the relative positions of oxygen atoms in the benzodioxepane fragment (expanded dioxane ring [1]) are accompanied by chemical features.
Our goal is to establish the structure of hydroxyl-containing 1,5-benzodioxepane analogs of chalcones using the physical research method SIAR (Synthetic Impulse and Aperture Radar) [2].
Based on the magnitude of the chemical shifts, it is impossible to draw any definite conclusions regarding the conformation of the conjugation chain present in the chalcone molecules. The assignment of the signals of olefin protons themselves is also not obvious, since the well-known assumption that in a weaker field absorbs the signal of a proton located in the β-position relative to ketone carbonyl does not have sufficient grounds.
When passing from solutions in CDCl3 to solutions of chalcones in C6D6, significant shifts are observed for individual proton signals in a weak field (positive SIAR effect) or in a strong (negative SIAR effect) field. In hydroxyl-containing chalcones [1], the signals of the methylene groups of the heterocyclic residue are shifted farthest into the strong field, and the greatest shifts are observed in the weak field for the signal of the hydroxyl proton.
SIAR values allow us to draw certain conclusions regarding the conformation of the conjugation chain in the molecule of 1,5-benzodioxepane chalcon. In doing so, we used the Connolly and Mac Grindla model [3] to associate the carbonyl group with the benzene molecule. According to this model, if a plane perpendicular to the
C = O bond line is drawn through the carbonyl oxygen atom, then protons located relative to this plane from the side of the carbonyl carbon atom experience negative SIAR, and protons located on the other side of the drawn plane experience positive SIAR. In the molecule of the studied chalcone, rotation around one of the single chemical bonds of the conjugation chain (bond a) is very difficult due to the formation of a strong intramolecular hydrogen bond. Therefore, four flat conformations can be proposed for the studied chalcon (scheme 1).
Using the atom-atom potential method, we found a change in the energy of a chalcone molecule during rotation around bonds b and d.
The corresponding energy scheme is shown in figure 1. Here, two coordinate axes correspond to rotation around the bonds b and d, and the third coordinate axis characterizes the total energy of the molecule. It can be seen from the figure that the ZZEE and ZZEZ conformations are most favorable, while the ZEEE and ZEEZ conformations have a maximum of energy associated with spatial difficulties arising between the olefin proton and the nearby proton of the 6-H benzene core. The implementation of a somewhat non-flat conformation, although it leads to a decrease in spatial noise, however, the energy is much larger than in the ZEEE and ZEEZ conformations. This is also confirmed by calculations with optimization of the geometry of the chalcone molecule using the iterative method for all possible plane conformations. The equality of energies for the conformation of ZZEE and ZZEZ suggests that chalcone molecules exist as a mixture of equal amounts of these conformers with a high, in the NMR time scale, transition rate between confirmations. An indirect confirmation of this is the absence of a noticeable increase in the intensity of the signals of aromatic protons of the heterocyclic fragment of the chalcone molecule in experiments on the Overhauser homonuclear effect [4–7] with irradiation at the HB frequency.
From the 1H NMR spectra [8], it follows that for one of the olefin protons of SIAR chalcones, they are either positive or close to zero, while for the other olefin proton, SIAR is always negative. According to the Connolly and Mac Grindla model, an olefin proton with positive SIAR should be located near the plane passing through the carbonyl oxygen atom perpendicular to the C = O bond line. In the most favorable conformations of ZZEE and ZZEZ, such a proton is the HB proton. Therefore, SIAR values can serve as the basis for assigning the signal of a given proton in the PMR spectrum. According to our data, in the studied chalcon, the HB proton absorbs in a weaker field than the HA protonThis conclusion is confirmed by the PMR spectrum of chalcones synthesized from deuterated 2-benzyloxyacetophenones [9]. Such chalcones, instead of the HA atom, contain a deuterium atom; in their PMR spectra there is no signal of a high-field olefin proton. It follows that the assumptions made earlier in the literature on the assignment of olefin proton signals in the PMR spectra of chalcones are confirmed.
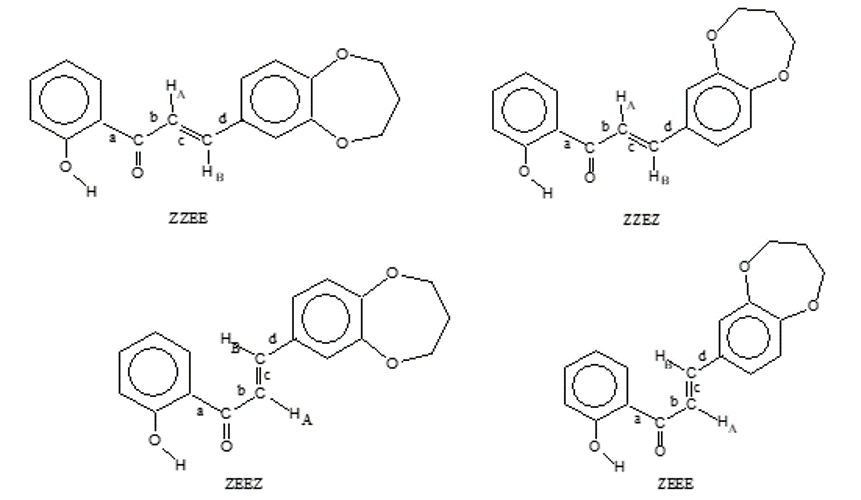
Scheme 1. Hydroxyl-containing 1,5-benzodioxepane chalcon.
Thus, the totality of the experiments performed allows us to state that all the studied 2-hydroxychalcones are a mixture of equal amounts of ZZEE and ZZEZ conformers in CDCI3 and C6D6, and in this weaker field they absorb HB olefin proton.
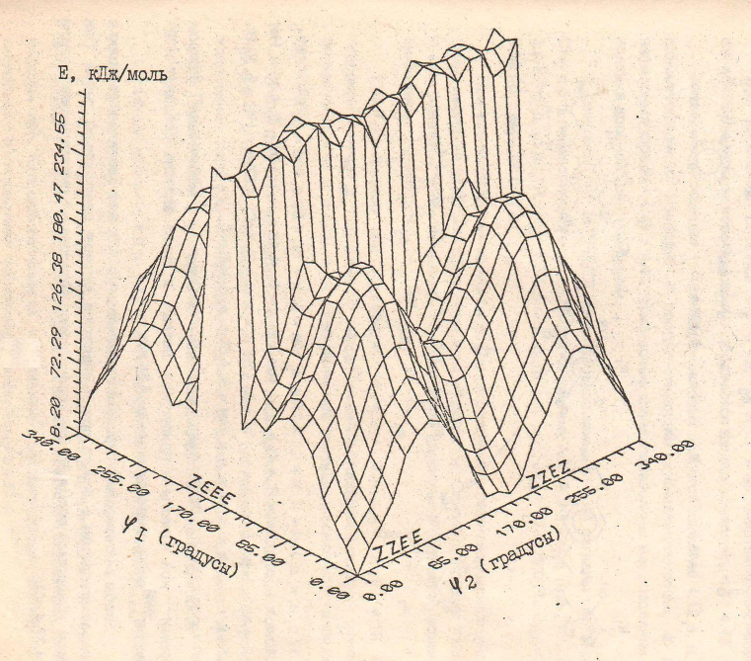
Figure 1. SIAR effect of 1,5-benzodioxepane chalcones.
(E, Z) — is the standard double bond of stereochemistry.
References
1. Ismailova G O, Uzahbergenov A A, Uzakbergenova Z D, 2016. The nature of the decay of molecules and the assessment of possible ways of fragmenting the structure of heterocyclic derivatives of chalcones using mass spectrometric analysis. Almanac of modern science and education. Tambov: Diploma. No. 2(104). P. 48-542. Baixiao Chen, Jianqi Wu, 2014. Synthetic Impulse and Aperture Radar (SIAR): A Novel Multi-Frequency MIMO Radar. Book. - 448 P. (Google Books.html)
3. Models of visual display of the structure. Three-dimensional structures 3D structures. www.abc.chemistry.bsu.by
4. Keeler J, 2010. Understanding NMR Spectroscopy. Wiley, No. 2. ISBN 978-0-470-74608-0
5. Akitt J W, Mann B E, 2000. NMR and Chemistry. Cheltenham, UK: Stanley Thornes Ltd, No. 4. 400 p. ISBN 0-7487-4344-8
6. Overhauser Albert W, 1953. Polarization of Nuclei in Metals. Physical Review, 92 (2): Р. 411-415. DOI:10.1103 / Phys Rev. 92.411
7. Voronov V K, Podoplelov A V, 2005. Modern Physics: Textbook. - M .: Kom Book, Ch. 6. The structure and dynamics of molecules, p. 6.1. Magnetic resonance, p.p. 6.1.12. Overhauser Effect, P. 354-359. ISBN 5-484-00058-0
8. Gabriele F, Giuliani and Samuel A, 2012. Werner Albert Warner Overhauser . Physics Today, Vol. 65. P. 69. DOI:10.1063 / PT.3.1758
9. Aitmambetov A, 1994. Synthetic analogues of natural flavonoids and flavolignans. PhD Thesis, Kiev
