Electric vehicles represent one of the most promising and rapidly developing categories of vehicles in the modern world. Unlike traditional cars with internal combustion engines that run on gasoline or diesel fuel, electric vehicles use electrical energy stored in batteries to power electric motors. This shift to electric transport is driven by several factors, including the need to reduce greenhouse gas emissions, reduce dependence on fossil fuels, and the desire for more sustainable methods of transportation. The main component of an electric car is a rechargeable battery, which provides it with energy. By far the most common are lithium-ion batteries, which have a high energy density and a long service life. These batteries allow electric vehicles to travel significant distances on a single charge, making them convenient for everyday use. However, over time, the efficiency of the batteries decreases, which leads to the need to replace them. In this context, it is important to understand not only the principles of operation of electric vehicles, but also the features of their batteries.
Electric vehicles can be classified into several types: fully electric, hybrid and plug-in hybrids. Fully electric cars run exclusively on electricity and do not have an internal combustion engine. Hybrid cars combine both an electric motor and an internal combustion engine, which allows them to take advantage of both types. Plug-in hybrids can be charged from the mains and provide greater flexibility in use. The principle of operation of an electric vehicle is based on the conversion of electrical energy into mechanical energy. Electric motors convert electricity from batteries into the rotational motion required to move the car. During operation, electric motors demonstrate high efficiency — up to 95%, which is significantly higher compared to internal combustion engines, which lose a significant amount of energy in the form of heat.
With the increasing number of electric vehicles on the roads, the issue of accessibility and convenience of charging is becoming important for both car owners and charging station operators. An electric vehicle charging station is a device that transfers electrical energy from a power source to a car battery via a special cable. The principle of operation of the charging station is based on the conversion of electric current, which can be either alternating or constant. When using alternating current, the conversion takes place inside the electric vehicle itself, where the built-in inverter converts it to direct current to charge the battery. In the case of DC charging stations, this process is carried out directly at the station level, which significantly reduces the charging time.
The charging process usually involves several steps: connecting the cable to the port of the electric vehicle, converting the current, monitoring the charging process and completing the charging. Modern charging stations are equipped with intelligent control systems that regulate the current and voltage, ensuring the safety and efficiency of the process. They can also exchange data with the car, which allows you to optimize the charging process depending on the state of the battery. The installation of charging stations requires careful planning and consideration of many factors, such as location, availability of the electrical network, types of connectors and power of chargers. It is important to take into account the needs of users: the availability of fast charging can be a decisive factor when choosing an installation location. In this regard, the development of standards and recommendations for the installation of charging stations becomes necessary to ensure their reliable operation and safety [1, 2].
At the moment, there are three main types of batteries used in modern devices:
Lead-acid batteries are a type of battery that has become widespread due to its moderate cost, good life (more than five hundred cycles) and high specific power. They are used in various fields, such as starter batteries in vehicles, emergency power sources, as well as backup energy sources.
Lead-acid batteries consist of electrode plates made of a lead lattice filled with PbSO4 (lead sulfate) with a binding material. The current carrying strips and battery terminals are also made of lead.
The principle of operation of lead-acid batteries is based on the reactions of lead (cathode) and lead dioxide (anode) in an aqueous solution of H2SO4.
When an electric power consumer is connected, a chemical reaction occurs in which lead oxide and sulfuric acid react. Lead oxidation occurs. When the battery is discharged, lead oxide is reduced at the cathode, and lead oxidation occurs at the anode. When the battery is charged, the reverse process occurs and water is released in reaction with a solution of sulfuric acid. It can boil due to adverse reactions and lead to battery failure and reduced service life.
This type of battery has the following advantages: time-tested old technology, very low self-discharge, minimal maintenance, the ability to provide high current output if necessary, ease of production and low cost.
The disadvantages include: storage only in a charged state, large weight and size, sensitivity to negative batteries, a limited number of discharge cycles, environmentally friendly temperatures, low energy consumption compared to other types are dangerous due to lead compounds.
Nickel-cadmium batteries have been used for more than 15 years. This is almost three times more than that of lead–acid batteries, whose service life is about 5 years, and in case of improper operation associated with boiling off the electrolyte — no more than 3 years. Nickel-cadmium batteries are reliable enough and they are not characterized by boiling, unlike lead-acid batteries. Also, this type of battery is easier to maintain. Nickel-cadmium batteries are about a quarter more expensive than lead-acid batteries, but they are more durable.
There are two electrodes in the battery: nickel and cadmium. The nickel electrode consists of a mixture of nickel hydroxide with a conductive material, and the cadmium electrode is a steel mesh with cadmium pressed into it. There is a space between the electrodes that is filled with alkali. A chemical reaction occurs with nickel, cadmium and water hydroxides, which is reversible.
Nickel–metal hydride batteries were produced in the 1980s to replace nickel-cadmium batteries. But nickel-cadmium batteries are still used despite the fact that they are gradually being abandoned, as there is a better alternative.
Lithium-ion batteries, in this type of battery, lithium ions move between the cathode and the anode, creating a flow of electricity. They have the following advantages: they operate at temperatures from -20 to +50°C, high density of stored energy and discharge currents, constant readiness for efficient operation.
However, despite these advantages, lithium-ion batteries have significant disadvantages. The first is that they have low performance at subzero temperatures. To overcome this disadvantage, various battery heating systems are used. For example, a heat-protected lithium-ion battery with heating elements, which allows the battery to operate at low temperatures. The second is aging, which causes loss of residual capacity, and the number of charge–discharge cycles does not correlate with the life of a lithium-ion battery. Temperature and age have a greater influence: with short and continuous charge-discharge cycles and cooling or heating, in cold conditions, a lithium-ion battery can withstand from 1000 cycles to 3000 cycles.
After a few years, the used batteries of electric vehicles can no longer meet the requirements of electric vehicles, for example, such as the range of travel on a single charge. Some manufacturers change the battery of electric vehicles under warranty when the residual capacity reaches 60% or lower, but there are examples of replacement at 60-70%.
The main part of the installation for optimizing the operation of electric charging stations of electric vehicles are used rechargeable batteries of electric vehicles. According to the regulations of car service stations, the battery is replaced when the residual capacity reaches 60% or lower, this capacity value is not enough for full operation of the electric vehicle, but the battery life is exhausted by less than half, which is enough for the installation to work. This allows you not only to have a guaranteed battery supplier, but also to develop and use a standard battery installation and connection solution, since all cars use the same battery model [3].
Next, an installation solution was developed: the batteries are located in a special rack one above the other in three pieces, the vertical supports are 6 reinforced metal profiles with a height of no more than 1.7 meters. The profiles are mounted on a metal plate from below. Batteries are installed between horizontal reinforced metal profiles, which are mounted to vertical profiles using consoles, on a metal plate to simplify their replacement. The appearance of the racks is shown in Fig.1. All metal parts are treated against corrosion by hot-dip galvanizing.
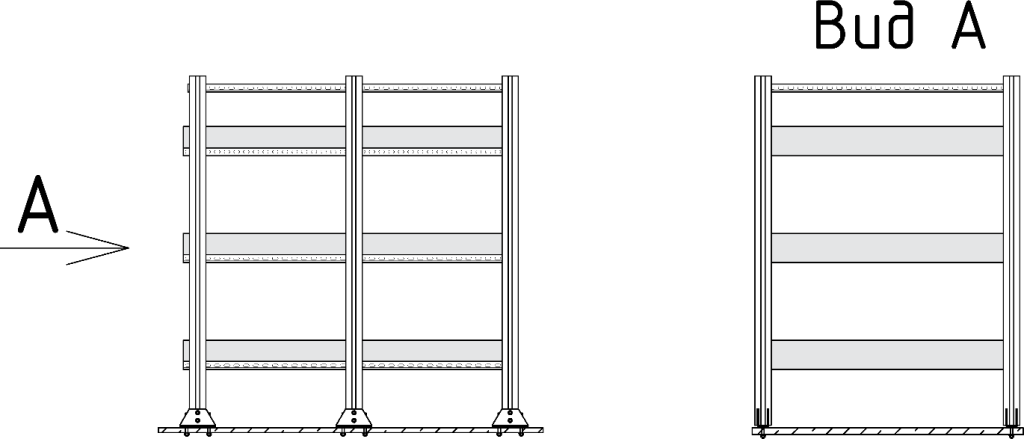
Figure 1. Racks with rechargeable batteries.
According to the Autostat agency, at the moment all passenger electric vehicles have an average battery capacity of 51.5 kWh. But given that the field of electric transport is rapidly developing, and the consumer has a clear request to increase the capacity of batteries so that it is possible to travel long distances on a single charge, the average capacity value will grow, approaching the value of 60 kWh.
With the expectation of an increase in the statistical average value of the capacity of new batteries, the capacity value of 39.4 kWh is approximately 66%. It follows from this that in order to fully charge an electric vehicle with a capacity value of 60 kWh, the operation of two used batteries is necessary. On average, 3 electric vehicles will be able to charge in 4 hours of operation, so 6 batteries are needed. This is the first criterion for determining the number of used batteries in the installation. The second criterion was the maximum power output from the charging station, and the amount of time during which this power will be output [4].
Based on these two criteria, it was decided that 2 racks or 6 used batteries are needed to optimize the operation of one EHS.

Figure 2. Charging station «FORA EZ-DC-1×50»
Figure 2 shows a block diagram of the distribution of electrical energy between two racks with rechargeable batteries belonging to one EHS. Such a solution may be typical, and depending on the number of EHS, the number of racks will change in accordance with the fact that 2 racks with rechargeable batteries are needed for one 50 kW charging station. To charge the used batteries, the rectifier of the electric charging station is used, since it is already designed for the parameters necessary for charging electric vehicle batteries.
From the point of view of thermal power engineering, the use of spent lithium-ion batteries allows not only to reduce the load on generating capacities, but also to increase the overall efficiency of the power system due to heat recovery. During the charging and discharging of batteries, heat is released, which is usually dissipated into the environment and lost. However, with the help of modern thermal management technologies, this heat can be used for space heating, water supply or even for industrial processes, which further increases the energy efficiency of the installation [5].
In addition, optimizing the operation of charging stations using such installations helps to reduce fluctuations in the power grid, providing a more stable and high-quality power supply. This is especially important in the context of the growing number of electric vehicles and, accordingly, the increasing load on the power grid.
Thus, the integration of spent lithium-ion batteries into the infrastructure of charging stations allows not only to improve the economic efficiency and environmental friendliness of the stations, but also to provide additional advantages in terms of thermal energy optimization, which makes this area very promising for further development and research.
References
1. Шуркалов, П. С. Возможности подзарядки электромобилей от установок на основе возобновляемых источников энергии / П. С. Шуркалов, М. Г. Тягунов // Вестник Московского энергетического института. Вестник МЭИ. – 2013. – № 5. – С. 061-066.2. Вахрушев, М. А. Анализ эффективности использования зарядных станций для электромобилей / М. А. Вахрушев // Столыпинский вестник. – 2022. – Т. 4, № 4.
3. RES-powered charging stations for electric vehicles / S. Volkov, V. Sidorova, A. Orlov, A. Ostashenkov // IOP Conference Series: Materials Science and Engineering, Krasnoyarsk, 20–21 ноября 2020 года / Krasnoyarsk Science and Technology City Hall.. Vol. 1047. – Krasnoyarsk, Russian Federation: IOP Publishing Ltd, 2021. – P. 12182.
4. Шакиров, М. А. Технология зарядных станций для электромобилей / М. А. Шакиров // Цифровое общество: научные инициативы и новые вызовы : Сборник научных трудов по материалам V Всероссийской научно-практической конференции, Москва, 17 апреля 2024 года. – Москва: ООО "Издательство "Экономическое образование", 2024. – С. 191-195.
5. Денисенко, Р. В. Зарядная станция для электротранспорта нового типа / Р. В. Денисенко // EUROPEAN RESEARCH: сборник статей победителей VII международной научно-практической конференции, Пенза, 07 декабря 2016 года. – Пенза: "Наука и Просвещение" (ИП Гуляев Г.Ю.), 2016. – С. 85-87.
